Plasmids
RAS and ERK BRET sensors were developed by replacing the fluorescent energy donor (ECFP or Turquoise-GL) and the fluorescent energy acceptor (YPet-M) of the EKAREV and RaichuEV-ras FRET probes27 with nanoLuciferase (nLuc28) and mNeonGreen (mNeonG29), respectively. Mammalian expression vector coding ERK (pEKAREV) and RAS (pRaichuEV-Ras) FRET probes were kindly provided by Dr Matsuda M (Kyoto University, Japan). The cDNA coding for nLuc and mNeonG were first synthetized (Genescript, Rijswijk, The Netherland) and then cloned in place of the fluorescent energy donor, between NotI and XbaI, in pEKAREV and pRaichuEV-Ras expression vectors.
The cDNA encoding the nLuc-HSF1 and mNeonG-HSF1 proteins were derived from rLuc-HSF1 and sYFP2-HSF1 expression vectors13, in which Renilla Luciferase II and sYFP2 groups were replaced by nLuc and mNeonG, respectively, between BamHI and EcoRI. The HSP90 expression vector was also described in Poque et al.13.
Similarly, the cDNA encoding the nLuc-PMLIII and mNeonG-SUMO1 proteins were derived from rLuc-PMLIII and YFP-SUMO1 expression vectors26, in which Renilla Luciferase and YFP were replaced by nLuc and mNeonG, respectively, between BamHI and EcoRI.
Reagents
As2O3 (A1010, 330 mM stock solution resuspended in 1 N NaOH) and MG132 (C2211, Z-Leu-Leu-Leu-al, 50 mM stock solution resuspended in DMSO) were from Sigma (Lyon, France). Phorbol-12-myristate-13-acetate (PMA) was acquired from Tocris (Bristol, UK), and Coelenterazine H from Nanolight Technology (Pinetop, AZ, USA).
Cell culture and transfections
We have used the SV-40 immortalized skin fibroblast line established from a 19-year-old female with xeroderma pigmentosum (XP), complementation group D, the XP6BE line30 supplied by the Coriell Institute (Camden, NJ). XP6BE fibroblasts were maintained in Dulbecco’s modified Eagle’s medium – high Glucose (DMEM) (D6429, Sigma) supplemented with 10% fetal bovine serum, 100 units mL-1 penicillin and streptomycin. HaCaT keratinocytes31 were maintained in Keratinocyte-SFM (Ref 17005-034; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with Bovine Pituitary Extract (BPE; Catalogue Number 13028-014, Gibco, Thermo Fisher Scientific, Waltham, MA, USA) & Human Recombinant EGF (catalog number 10450-13, Gibco, Thermo Fisher Scientific, Waltham, MA, USA) supplied with the kit Gibco™ Keratinocyte-SFM Supplement (Ref 37000015, Gibco, Thermo Fisher Scientific, Waltham, MA, USA). Twenty-four hours before transfection, cells were seeded at a density of 500,000 cells per well in 6-well dishes. Transient transfections were performed using polyethylenimine (PEI, linear, MW 25,000; catalogue number 23966 Polysciences, Inc., Warrington, PA, USA) with a PEI:DNA ratio of 4:1, as previously described32. For measurement of HSF1 activity, 0.1 µg of nLuc-HSF1 expression vector was co-transfected with 1.4 µg of mNeonG-HSF1 and 0.5 µg of HSP90 expression vectors. For measurement of RAS and ERK activities, 1 µg of pEKAREV or pRaichuEV-Ras BRET expression vectors were co-transfected with 1 µg of empty vector. For measurement of PML activity, 0.1 µg of nLuc-PMLIII expression vector was co-transfected with 1.9 µg of mNeonG-SUMO1. After overnight incubation, transfected cells were then detached, resuspended in DMEM w/o red phenol (Ref 21063-029, ThermoFisher scientific, Waltham, MA, USA) and replated at a density of 105 cells per well in 96-well white plates with clear bottoms (Greiner Bio one, Courtaboeuf, France) pre-treated with d-polylysine (Sigma, Lyon, France) for reading with the Tristar2 luminometer (Berthold Technologies, Bad Wildbad, Germany) or onto 12 mm diameter glass coverslips (Knittel Glass, Braunschweig, Germany) treated with d-polylysine for the reading with the SpectraPro 2300i spectrometer (Acton Optics, Acton, MA, USA) (see below). Cells were left in culture for 24 h before being processed for the BRET assay.
BRET measurements
Cells were sham-exposed for 24 h (i.e. the cells were cultivated in absence of RF-EMF) or exposed to the indicated RF-EMF exposure conditions for 24 h. During the last 18 h, 4 h or 15 min of either sham or RF-exposure, the cells were incubated with the indicated concentration of MG132, As2O3 or PMA respectively (Fig. 1A). Only one chemical was tested in each 96-well plate; however various concentrations of the same chemical compound were tested simultaneously in a single plate by injecting 10X stock-solutions pre-heated at 37 °C using a multi channel pipette. When indicated, mock-treatment were performed by injecting only the solvating agent into the cell culture medium (DMSO for MG132 and PMA or water for As2O3).To perform the chemical treatment, the plates were removed from the reverberating chamber (see below “Cells exposure, exposure set-up, and dosimetry” section of the Material and methods) and immediately docked on a Thermostat Plus microplate Peltier heater (Eppendorf, Hamburg, Germany) to keep the cells at 37 °C. The sham-exposed plates were treated the same way. Removing the plate from the reverberating chamber, dispatching the chemicals at the various concentrations in the cell culture media using a multi-channel pipette and replacing the cells into the reverberating chamber took less than 1 min. After completion of the remaining RF exposure, 5 µM Coelenterazine H was added to the cell culture medium and the BRET signal was immediately acquired using a TriStar2 LB942 microplate reader (Berthold Technologies, Bad Wildbad, Germany) pre-heated at 37 °C and equipped with emission filters centered at 515 ± 20 nm for mNeonG (Iacceptor) and 460 ± 20 nm for nLuc (Idonor). Alternatively, for real-time BRET measurement under RF-EMF exposure, 5 µM Coelenterazine H was added to the cell culture medium 10 min before the end of the RF-EMF exposure and the full BRET spectra were recorded remotely for the last 5 min of RF-EMF exposure and the 5 next min in absence of RF-EMF. Full BRET spectra were acquired using an optical fiber linked to an IsoPlane SCT-320 Imaging Spectrograph equipped with a BLAZE:400B back illuminated CCD camera system camera for recording the full visible spectrum (Teledyne France—Princeton Instruments, Lisses, France).
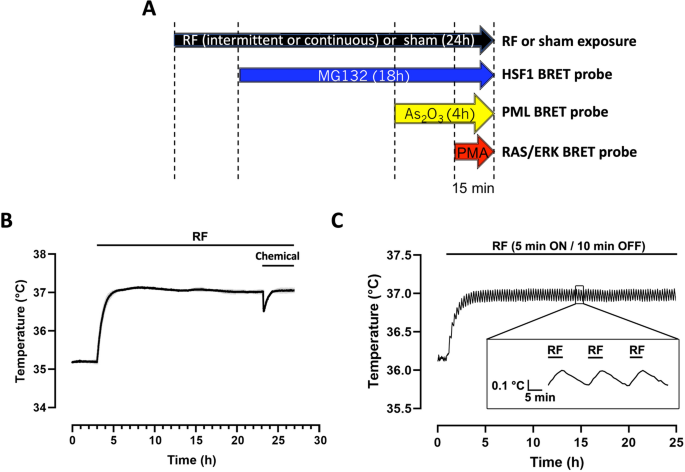
(A) Timeline of the RF-EMF exposure and chemical drugs addition in the cell culture medium. RF-EMF (or sham RF exposure) is applied for 24 h whatever the drug considered, that is injected for the indicated period. (B,C) Temperature variation in plates exposed to a continuous (B) or intermittent (C) 5G-modulated 3.5 GHz RF-EMF signal at 4W/kg. The temperature in the incubator was set to ensure cellular exposure at 37 °C and to compensate for the RF EMF-induced temperature increase at the onset of the exposure period. Drug injection induces a less than 0.5 °C transient drop in the cell culture temperature (B).
The BRET signal was determined by calculating the ratio of the emission intensity measured in the acceptor window (ImNeonG) over the emission intensity measured in the donor window (InLuc), according to Eq. (1)
$${\text{BRET}}=\frac{{I}_{\text{mNeonG}}}{{I}_{\text{nLuc}}}.$$
(1)
Due to the overlapping emission spectra of nLuc and mNeonG, a fraction of the light detected in the mNeonG filter originates from the luciferase emission, resulting in a contaminating signal33. In that configuration, the net BRET was therefore defined as the BRET ratio of cells co-expressing nLuc and mNeonG constructs minus the BRET ratio of cells expressing only the nLuc construct in the same experiment.
BRET data processing and statistical analysis
The GraphPad Prism v8.00 for Mac software (GraphPad Software, La Jolla, CA, USA) was used for plotting dose–response curves and performing statistical analyses. The size of the error bars indicates the S.D. within the data set. Sigmoidal dose–response curves were fitted using Eq. (2):
$${\text{Y}}=\mathrm{Bottom}+\frac{(Top-Bottom)}{1+{10}^{logEC50-X}},$$
(2)
where X is the logarithm of agonist concentration and Y the response; Bottom is the Y value at the bottom plateau and is taken as the measure of basal level of activation of the various probes; Top is the Y value at the top plateau and Top–Bottom is taken as the measure of the maximal efficacy of a given chemical treatment on each BRET probe; Log EC50 is the X value when the response is halfway between Bottom and Top (Supp. Fig. 1). The EC50 value represents therefore a measure of the apparent potency of the various chemical compounds to trigger the activation of their cognate BRET probe (Supp. Fig. 1). Potencies of chemicals to activate or inhibit the different probes are expressed as pEC50 ± S.E.M (standard error of the mean), that is equal to –log EC50.
The one sample Wilcoxon signed-rank test was used to assess the statistical significance against the null hypothesis of the differences calculated in each independent experiment between sham (no RF EMF condition) and RF-EMF exposure conditions for basal BRET, chemicals’ potencies and efficacies (hereafter denominated ΔBasal BRET, ΔpEC50, and ΔMax efficacy, respectively). The total number of independent experiment (n) performed for each experimental condition is indicated. One sham exposure was performed for each RF-EMF exposure condition. P-values less than 0.05 were considered as statistically significant.
Cells exposure, exposure set-up, and dosimetry
Cells were exposed for 24 h in 96-well tissue culture plates (TCP) at SAR levels of 0.25, 1, and 4 W/kg. Intermittent exposure (5 min ON/10 min OFF) at the same average SAR level as with the continuous wave (CW) mode was implemented to mimic actual real-life exposure and help detect potential nonthermal bioeffects. RF EMF sham exposures were also performed under identical experimental conditions but with the generator turned off, i.e. at SAR equal to 0 W/kg. A novel exposure system, recently designed and characterized, was used for the first time for cells exposures to 5G-modulated 3.5 GHz signals34. The system was based on a cell culture incubator that allowed maintaining the desired biological conditions of 37 °C and 5% CO2. Comprehensive characterization of the system, through experimental measurements and numerical simulations has been described in detail elsewhere34. Briefly, a 150-l incubator (BINDER Gmbh, Tullingen, Germany), made of stainless-steel walls, was used as a reverberation chamber, i.e. a metallic large, closed cavity, with a high Q-factor, where a statistically homogeneous, randomly polarized, and isotropic field distribution was achieved via mechanical stirring of the field components35. Electromagnetic signal at 3.5 GHz was delivered to the biological samples through a printed patch antenna. A plastic holder with five levels was used to accommodate and simultaneously expose ten TCPs of 6- or 96- wells i.e. two per holder level. Each well of the 6- and 96- well TCPs was filled with 2 ml and 200 µl of cell culture medium, respectively. To ensure experimental reproducibility during exposure, the incubator was loaded with the same configuration used for the electromagnetic characterization, i.e. four and six 6 -and 96-well TCPs, respectively, due to the high SAR dependence on the chamber load.
The signal generation unit, composed of a RF signal generator (SMBV100A, Rohde & Schwarz, Munich, Germany), a 45-dB gain amplifier (Mini-circuits, ZHL-16W-43 + , NY, USA), a power circulator (Pasternack, PE83CR1005, CA, USA), and a bidirectional coupler (Mini-circuits, ZGBDC30-372HP + , NY, USA), was located outside the incubator. In addition, to ensure the continuous monitoring of the desired input power into the chamber, incident and reflected powers were monitored with a power meter (Agilent N1912A, USA) connected to the bidirectional coupler.
Local SAR was experimentally retrieved through temperature measurements of the RF-EMF induced heating recorded with a fiber-optic probe (Luxtron One, Lumasense Technologies, CA, USA). Measured SAR in the 96-well TCP was around 1 W/kg per watt antenna input power. To validate our systems, numerical simulations were performed using the finite difference time domain (FDTD)-based electromagnetic methodology36. The results of simulations were averaged over 50 positions of the stirrer, corresponding to a complete rotation. Although numerical simulations might not guarantee the absence of hotspots at specific locations of the exposed wells, the continuous stirring of the field components via the mechanical rotation of the metallic stirrer ensured the achievement of a good SAR homogeneity with variation within 30%. Overall, we showed that experimental and numerical SARs were in good agreement with differences < 30% considering the standard deviation that is compliant with ICNIRP guidelines3. According to measured and simulated values normalized to 1 W, incident power during biological exposure was adjusted to obtain required exposure levels of 0.25, 1, and 4 W/kg in a 96-well tissue culture plate. Measurements of the induced temperature elevation of the exposed medium were also performed using the Luxtron probe (Lumasense) under the specific cellular exposure condition of the study, showing a temperature increase of 1.7 °C at 4 W/kg, 0.7 °C at 1 W/kg and a negligeable temperature increase below 0.1 °C at 0.25W/kg using a continuous RF exposure, and a temperature increase of 0.8 °C at 4 W/kg, 0.3 °C at 1 W/kg and less than 0.1 °C at 0.25 W/kg using an intermittent (5 min ON, 10 min OFF) RF exposure. The temperature of the incubator was decreased accordingly to maintain the biological samples at 37 °C. Temperature stability of cell cultures at the bottom of the culture wells during the whole RF sessions at the various SAR levels was carefully assessed in a set of separate plates (See Fig. 1B,C for typical temperature traces obtained at 4 W/kg under continuous and intermittent exposure conditions, respectively). As expected, the temperature of the cell culture exposed to the intermittent signal is slightly waving (Fig. 1C). Of note, injection of the chemical triggered a transient drop in cell culture temperature by less than 0.5 °C as exemplified in Fig. 1B.
