This open-label, randomised, crossover, clinical study received favourable opinion (equivalent to ethics approval) from the Office for Research Ethics Committees Northern Ireland (ORECNI) Health and Social Care Research Ethics Committee A (reference number 21/NI/0144) prior to study commencement. The study was performed in accordance with ethical principles set forth in the Declaration of Helsinki and was compliant with the principles and requirements of International Council for Harmonisation Good Clinical Practice (ICH-GCP), the European Union Clinical Trials Directive, and applicable local regulatory requirements. The study was performed at a single clinical site in Belfast, Northern Ireland (UK) and was registered in the ClinicalTrials.gov repository (NCT05459857). Twenty-four (24) male and female healthy adult smokers participated in this study. Subjects attended the clinic site on two separate occasions: a screening visit and a 5-day confinement period. After completing the study, a follow-up telephone call was conducted for all subjects no longer than 1 week after the end of their confinement period. All subjects provided written informed consent prior to the commencement of any study procedures, including screening assessments.
Study subjects
In total, 51 subjects were screened for participation in this study; 27 subjects either failed screening or were not needed for study participation (i.e., they were study reserves but were not required to take part in the study). Of the 24 enrolled subjects, 23 completed the study; one subject withdrew from the study for personal reasons.
Inclusion criteria were that subjects were healthy adults aged 21–65 years inclusive at screening; self-reported smoking at least 10 manufactured combustible (menthol or non-menthol) cigarettes per day (CPD) for at least 12 months prior to screening; had a urine cotinine ≥ 500 ng/mL at screening; had an exhaled carbon monoxide (eCO) level > 10 parts per million (ppm) at screening; if female and of childbearing potential were using at least one approved form of contraception; if female and of non-childbearing potential had undergone a sterilisation procedure at least 6 months prior to check-in or was postmenopausal with amenorrhea (verified by measuring follicle-stimulating hormone (FSH) levels) for at least 1 year prior to check-in; if a non-vasectomised male agreed to use a condom with spermicide or abstain from intercourse for the duration of the study and extending up to 90 days post-study; if male agreed not to donate sperm for duration of the study and extending up to 90 days post-study; was willing comply with the requirements of the study, including a willingness to use the study HTPs; and provided voluntary consent to participate in this study, which was documented by signing of the signed informed consent form.
Subjects were not allowed to enter the study if any exclusion criteria were met. The main exclusion criteria were a history or presence of clinically significant disease or disorder that, in the opinion of the Investigator, would have jeopardised the safety of the subject or impacted the validity of the study results; had a clinically significant abnormal finding on the physical examination, medical history, vital signs, electrocardiogram, or clinical laboratory results; had an acute illness (e.g., upper respiratory tract infection, viral infection) requiring treatment within 14 days prior to Check-in; systolic blood pressure (BP) < 90 mmHg or > 150 mmHg, diastolic BP < 40 mmHg or > 95 mmHg, or heart rate (HR) < 40 bpm or > 99 bpm at screening; estimated creatinine clearance (using the Cockcroft Gault equation) < 70 ml/min at screening; used medications known to interact with cytochrome P450 2A6 within 3 months prior to check in and throughout the study; used inhalers to treat any medical condition within 3 months prior to check in and throughout the study; used prescription or over-the-counter bronchodilator medication (e.g., inhaled or oral β-agonists) for treatment of any illness within 12 months prior to check in and throughout the study; was allergic to or could not tolerate menthol flavouring agents; had used any prescription smoking cessation treatments, including, but not limited to, varenicline (Chantix®) or bupropion (Zyban®) within 3 months prior to check in; was planning to quit smoking during the study or within the next 3 months or was postponing a quit attempt in order to participate in the study; or had donated blood or blood products (including plasma), had significant blood loss, or received whole blood or a blood product transfusion within 90 days prior to check in.
Study products
The Pulze HTS, which comprises the Pulze HTP device and iD sticks, generates a nicotine-containing aerosol by heating consumable sticks containing reconstituted tobacco using an electrically powered heating device as its heating source. This heating creates an inhalable aerosol to deliver nicotine via the lungs. The device can be operated in two different user-selected modes (‘standard’ and ‘eco’ modes) in which the device heats to temperatures of 345 °C and 315 °C, respectively. In this study, subjects only used the Pulze HTP in ‘standard’ mode. Once switched on the Pulze HTS device is operational for 4 min, which allows users to take approximately 8–9 puffs from a single iD stick.
The tobacco sticks contain a portion of reconstituted tobacco and other non-tobacco components. Three test iD consumables (reconstituted tobacco sticks used with the Pulze HTS device) were assessed in this study; Intense American Blend, Regular American Blend, and Regular Menthol. The aerosol from the Intense and Regular American Blend sticks has a tobacco aroma, while the aerosol from the Regular Menthol sticks has a menthol aroma due to the inclusion of a menthol-flavoured monoacetate filter at the mouth-end of the stick. The reconstituted tobacco in each of the Pulze iD consumables used in this study has a target specification of 4.6 mg of nicotine per stick, and different aerosol yields of nicotine are achieved by the use of different filters in each of the different iD consumables. When used under International Organization for Standardization (ISO) intense machine puffing conditions (55 ml bell-shaped puff over 2 s with a 30-s interpuff interval and with filter vents blocked)72, the Intense American Blend variant produces a higher yield of nicotine (mean yield 1.09 mg per stick) than either the Regular American Blend or Regular Menthol variants (mean yields both 0.64 mg per stick).
For use as a comparator product, all subjects provided their usual brand of cigarette.
Randomisation
Subjects who completed the study screening assessments were assigned a unique randomisation identification number. Subsequently, each subject, based on the identification number, were assigned to use the study products according to one of four product sequences, which were prepared by Celerion, Inc. These sequences were ABCD, BDAC, CADB and DCBA where A is the Pulze HTS with iD Intense American Blend sticks, B is the Pulze HTS with iD Regular American Blend sticks, C is the Pulze HTS with iD Regular Menthol sticks, and D is subjects’ usual brand cigarettes.
Study procedures
This study was a randomised, open label, crossover, confinement study in 24 male and female cigarette smokers. The study assessed 3 test products and a cigarette comparator and evaluated nicotine pharmacokinetics, subjective effects, puff topography and product safety. An overview of the study design is presented in Fig. 2.
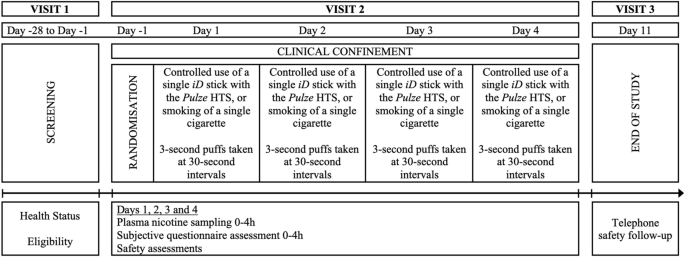
At Visit 1 (screening), which took place within 27 days prior to study procedures on Day −1, subjects underwent numerous assessments to check their eligibility to participate in the study, to review their health status, and to assess their nicotine consumption habits. Screening procedures included a physical examination (including oral cavity and oropharynx), vital signs, electrocardiogram, body mass index (BMI), clinical laboratory tests (hematology, serum chemistry, and urinalysis), serology, urine/saliva drug, urine/breath alcohol, cotinine screen, eCO, and pregnancy and FSH tests (for females as appropriate). If requested, subjects were offered smoking cessation advice and contact information for a smoking cessation support service.
Visit 2 was a 5-day confinement period; subjects who successfully completed the screening procedures and met all the inclusion criteria and none of the exclusion criteria were eligible to check in to the clinical site for the confinement period. Subjects checked into the clinic on Day −1 and remained at the clinic until Day 4 for daily study product use, nicotine pharmacokinetics sampling, subjective questionnaire assessments, puff topography evaluation, and safety assessments. On Day −1, following eligibility confirmation, subjects undertook a familiarisation session with the study HTPs and the questionnaires. The site’s clinical team explained how the Pulze HTS was to be used. Subjects had the opportunity to see the products/devices and packaging and participated in a product trial in which they consumed one iD stick, of a flavour chosen by each subject, using the Pulze HTS. An explanation of how the questionnaires were to be administered to the subjects was given. After the familiarisation session and completion of check-in procedures, subjects were allowed to smoke their own cigarettes ad libitum but abstained from the use of any tobacco- or nicotine-containing products for at least 12 h prior to the start of the controlled product use session on the morning of Day 1. In the morning of Day 1, after pre-use assessments and confirmation of eligibility, the subjects were randomised to 1 of 4 product sequences and then provided a single product of the study product in the sequence to which they had been randomised. On Days 1 through 4, subjects used the assigned study product under controlled conditions (i.e., completely used a single iD tobacco consumable HTP stick or smoked a single cigarette), with puffs taken at 30-s intervals and puffs 3 s in duration. Blood samples for nicotine assessment were collected 5 min prior to initiating product use and at 2, 4, 6, 8, 10, 15, 30, 45, 60, 120, and 240 min following the start of study product use. Subjective effects questionnaires were administered to the subjects at defined intervals throughout the day, and safety was also monitored throughout the day. For all subjects, meals and snacks were provided at the appropriate times during confinement at the clinic site. Each meal and/or snack served at the site was standardised, was similar in caloric content and composition, and was taken at approximately the same time on each day. When confined at the clinic site, subjects were required to fast from all food and drink except water between meals and snacks.
On Days 1 through 4, following the 4-h pharmacokinetic blood collection, subjects started a 4-h ad libitum product use session (no limits on cigarette or HTP consumption) with the same study product as that used during the morning controlled use session. Puff topography assessments were also made in the study but are not reported in this manuscript, and no blood samples were taken in this period for nicotine pharmacokinetic analyses. After completion of the ad libitum use session, subjects were allowed to smoke their own cigarettes ad libitum until at least 12 h prior to the start of the morning controlled product use session scheduled on the following day. On Day 4, following completion of study assessments, subjects were allowed to smoke their own cigarettes and left the clinic after completing all final check-out requirements.
A follow-up telephone call (Visit 3) was made by the clinic in an attempt to contact all subjects who used at least one study product (including subjects who terminated the study early) using their standard procedures approximately 7 days after the final product use to determine if any adverse events (AEs) had occurred since the last study visit.
Nicotine pharmacokinetics
To determine blood plasma nicotine concentrations during and after use of the study products, blood samples (approximately 4 ml) were collected through an indwelling venous catheter at the time-points described above. Blood samples were drawn into dipotassium ethylenediaminetetraacetic acid (K2EDTA) vacutainer tubes via an intravenous catheter port and the plasma fraction separated off by centrifugation and pipetting. Plasma nicotine was analysed by liquid chromatography-tandem mass spectrometry (LC–MS/MS) at Celerion Bioanalytical Services (Lincoln, Nebraska, USA) using a validated analytical method with appropriate quality controls according to the Food and Drug Administration (FDA) Guidance for Industry (Title 21 Code of Federal Regulations Part 58). Processing of samples was completed by a non-tobacco user. The lower limit of quantification of plasma nicotine using the analytical method was 0.2 ng/ml.
Subjective effects assessments
The Intent to Use [100 mm visual analog scale (VAS)], Urge to Smoke (VAS), and Product Evaluation Scale (7-point scale55) questionnaires were completed using a computerised tablet device. All relevant software specific to the electronic questionnaires were provided by IVR Clinical Concepts (IVRCC; Saratoga Springs, New York, USA). The Urge to Smoke questionnaire was completed at Time 0 (pre-product use) and at 4, 8, 15, 45, 60, 120, and 240 min relative to the start of product use on Days 1, 2, 3, and 4. The Intent to Use questionnaire was completed at 240 min following the start of study product use on Days 1, 2, 3, and 4. The 21-item Production Evaluation Scale (PES) questionnaire was completed at 240 min following the start of study product use on each of Days 1, 2, 3, and 4. PES subscale scores in the domains of satisfaction (items 1, 2, 3, and 12), psychological reward (items 4, 5, 6, 7, and 8), aversion (items 9, 10, 16, and 18), and relief (items 11, 13, 14, 15, and reversed for 20), were generated from the individual items as described previously55.
Statistical analyses
The safety population comprised all subjects who had successfully completed eligibility requirements after checking in to the clinic site and used at least one study product. The outcomes population was a subset of the safety population and consisted of subjects who used a study product and had evaluable nicotine pharmacokinetics or subjective effects data. This population was used in the summary and analysis of all data presented in this paper.
Due to this being the first study assessing nicotine pharmacokinetics and subjective effects of the Pulze HTS, no sample size calculations could be performed. However, a sample size of 24 subjects was deemed adequate to meet the study objectives and this is in line with similar study designs in the literature46,56,68.
Demographics
Descriptive statistics are reported for continuous variables (age, weight, height, and BMI) and frequency counts were tabulated for categorical demographics variables (sex, ethnicity, and race). Descriptive statistics are also provided for smoking history variables (cigarettes smoked per day and number of years smoking).
Nicotine pharmacokinetics
Unadjusted plasma nicotine concentrations that were below the limit of quantitation (BLQ) were set to one-half of the lower limit of quantitation (LLOQ) for the calculation of descriptive statistics. Individual plasma nicotine concentrations were adjusted for baseline nicotine levels (“baseline-adjusted”) and all pharmacokinetic parameters were calculated based on the adjusted concentrations. Baseline adjustment was performed by subtraction of the pre-existing nicotine concentration from each nicotine concentration obtained after test product administration in that period/day for each subject using the following equation:
$${\text{C}}_{{\text{t}}} \, = {\text{ C}}_{{{\text{t}}\;{\text{unadjusted}}}} {-} \, \left[ {{\text{C}}_{0} \cdot{\text{ e}}^{{ – {\text{Kel}}\cdot{\text{t1}}}} } \right]$$
where Ct is the adjusted concentration at time t, Ct unadjusted is the observed concentration at time t, C0 is the pre-product use concentration (−5 min), Kel = ln(2)/t½, t½ is 2 h (approximate nicotine half-life), t is the actual sampling time since product administration, and t1 is the actual sampling time since the time of the pre-product use sample. After correction for pre-product use values, negative values were assigned a value of zero and all other values obtained were reported as is even if these values were BLQ.
SAS® software (Version 9.4) was used for data presentation and summarisation including descriptive statistics, statistical analyses, summary tables, graphs, and data listings. Descriptive statistics were generated for plasma nicotine concentrations and nicotine pharmacokinetic parameters by study product for all subjects, including sample size (n), arithmetic mean (mean), SD, coefficient of variation (CV%), standard error of the mean (SEM), minimum, median, and maximum at each nominal time-point. In addition, geometric mean, and geometric CV%, are provided for the Cmax (maximum plasma nicotine concentration) and AUC (area under the plasma nicotine concentration–time curve) parameters. Mean concentration–time profiles are presented on linear scales. Missing data were treated as missing, and no imputation was conducted.
A linear mixed-effects model for analysis of variance (ANOVA) was performed on the natural log-transformed pharmacokinetic parameters Cmax and AUCt following the morning product use session on each of Days 1, 2, 3, and 4. The model included sequence, product, and study period as fixed effects and subject-nested-within-sequence as a random effect. Geometric least-squares means (LSM), and 95% confidence intervals (CIs), are provided for the pharmacokinetic parameters Cmax and AUCt by study product. Geometric LSM ratios, 95% CIs of the geometric LSM ratios, and p-values are provided for the product comparisons of Cmax and AUCt. The comparisons of interest included each of the products compared to each other. The above statistical analyses were performed using SAS® PROC MIXED.
A non-parametric analysis (Wilcoxon Signed Rank test) was performed for the comparisons of Tmax (time to maximum plasma nicotine concentration) between each of the study products. The median difference and 95% CI of the difference are presented for each comparison. The CIs were constructed using Walsh Averages and the appropriate quantile of the Wilcoxon Signed Rank Test statistic.
Subjective effects
Urge to smoke
The derived parameters Emax (maximum reduction in urge to smoke), TEmax (time of the maximum reduction in urge to smoke), and AUEC0–240 (area under the time-effect curve between time zero and 240 min), are listed by subject and summarised by product using descriptive statistics, including n, mean, SD, CV%, SEM, minimum, Q1, median, Q3, maximum, and 95% CI. A linear mixed-effects model for ANOVA was used to compare urge to smoke parameters without data transformation; the mixed model includes product sequence, period, and product as fixed effects and subject nested within product sequence as a random effect. LSM and 95% CIs are provided for the Emax and AUEC0–240 by product. LSM difference, 95% CIs of the LSM difference, and p-values are provided for the product comparisons for Emax and AUEC0–240. The comparisons of interest included each of the products compared to each other.
Product evaluation scale
Descriptive statistics for the composite (satisfaction, psychological reward, aversion and relief) and individual (ease of use, comfort using in public and dependence concerns) PES factor scores55 are provided by product.
Intent to use
Descriptive statistics for the VAS raw score and bipolar score are summarised.
Safety assessments
Safety was monitored through physical examination (symptom-driven), vital signs measurements, electrocardiograms, and clinical laboratory tests (serum chemistry, hematology, and urinalysis). Adverse event (AE) information was also collected throughout the study. AEs [including serious AEs (SAEs)] were recorded from the start of the first product used until the end-of-study telephone call. Severity/intensity were graded as mild, moderate, or severe, and AEs were also assessed as unlikely, possibly, or probably related to the study product by the investigator.
