Introduction
Ulcerative colitis (UC) and Crohn’s disease (CD) are the main forms of inflammatory bowel disease (IBD). Both have multifactorial causes and a wide spectrum of clinical manifestations and complications, with the main therapeutic strategies being the control of symptoms and intestinal inflammatory activity.1–4 The highest prevalence of the disease is described in North America, with ranges of 44 to 201/100,000 for CD and 37 to 238/100,000 for UC, and in Europe, where the respective ranges are 8 to 214/100,000 and 21 to 294/100,000.5–7 Over recent years, some regions of Asia and Latin America, previously considered to have a low incidence and prevalence of IBD, have reported an increase in the number of cases.8–12 A systematic literature review of articles published in Latin American countries reported an IBD prevalence ranging from 15.0 to 24.1/100,000 for UC and 2.4 to 14.1/100,000 for CD. Of the 18 studies analyzed, 13 were from Brazil, where a significant increase in IBD incidence and prevalence was found in the last 21 years. Northeast Brazil, which harbors approximately one-third of the country’s population and has the lowest human development index of all the regions of Brazil, was covered by only two of the studies, and in one of them, the prevalence of IBD was estimated at 12 per 100,000 inhabitants.10
The increased incidence of cases in these regions may be multifactorial. For instance, greater urbanization and industrialization can result in changes in dietary patterns that may alter the microbiota and the adaptive immunity involved in the pathophysiology of the disease. Other factors that may explain this increase include greater exposure to smoking, less breastfeeding, greater exposure to antibiotics, better hygiene and sanitation, and greater access to the health system and complementary exams.13 Brazil has continental dimensions, with important differences in socioeconomic conditions, urbanization, industrialization, and ethnicity among the regions of the country. The latter may affect the clinical–epidemiological profile of IBD, while access to diagnosis and new therapies in poorer regions may affect the diagnosis times and clinical evolution patterns of the disease.
The limited number of publications relating to clinical cohort studies in Northeast Brazil renders analysis of this region difficult. In this study, we aimed to identify the clinical and epidemiological profile of patients with IBD treated at reference centers in three states of Northeast Brazil.
Materials and Methods
Study Design, Participants, and Ethical Approval
This was a prospective study involving patients recruited from January 2020 to December 2021 who were followed up at outpatient clinics of hospital units specializing in the care of patients with IBD in the capitals of three states of Northeast Brazil, namely, Pernambuco, Paraíba, and Rio Grande do Norte. The patients were consecutively included in the study as they were attended by the authors of the articles in specialized outpatient clinics and after signing the informed consent form. Their current and past medical history was then entered into the research database. Pernambuco, the largest state, has approximately 9 million inhabitants and three specialized centers—The Clinical Hospital of the Federal University of Pernambuco, Pernambuco State University, and Barão de Lucena Hospital. In the state of Paraíba, which has a population of approximately 4 million, the study was conducted at the Lauro Wanderley University Hospital, Federal University of Paraíba. Meanwhile, in the state of Rio Grande do Norte, with a population of approximately 3.5 million, the cohort study was conducted at the Onofre Lopes University Hospital of the Federal University of Rio Grande do Norte.
The diagnosis of IBD (CD and UC) was based on the presence of clinical, radiological, endoscopic, and pathological manifestations.
The study complied with the Declaration of Helsinki and was approved by the Federal University of Pernambuco Research Ethics Committee (CAAE 11844919.0.0000.8807). All the patients were informed about the study and were enrolled after signing an informed consent form. Their treatment was not affected if they refused to participate in the study.
Study Variables
An application was specifically developed to gather cohort information and systematize the clinical epidemiological data to be analyzed at the time of care and the inclusion of patients. The demographic and social variables recorded were sex, age, race, family income, and education. Information regarding a family history of IBD, previous appendectomy, and smoking habits was also obtained. The clinical variables included the location of the disease, its behavior (Montreal classification),14 extraintestinal manifestations, the need for surgical procedures, and the pharmacological treatment instituted. The interval between the onset of symptoms and definitive diagnosis was recorded. One suspected case of spondyloarthritis was evaluated using the questionnaire of Queiro et al.15 Axial spondyloarthritis was suspected when at least two of the following symptoms were present for more than 3 months: A history of lower back pain, morning lumbar stiffness for more than 30 minutes, and night awakening due to pain. Peripheral spondylitis was suspected when at least two of the following symptoms were present for more than 3 months: A history of peripheral joint pain, morning stiffness for more than 30 minutes, and joint swelling.15 The incidence rates of IBD diagnosis for each 5-year period were also evaluated.
Statistical Analysis
The absolute and relative frequencies of the variables are expressed as percentages. Continuous data were subsequently tested using the Shapiro–Wilk test to assess the assumption of normality. For data meeting the assumption of normality, the averages were evaluated with the Student’s t-test; otherwise, the Mann–Whitney test was used to evaluate the medians of the variables. To assess the statistical significance of the results for the qualitative data, the chi-square test with Yates’ correction was used when indicated; when necessary, Fisher’s exact test was used. A p-value <0.05 was considered significant.
Results
IBD Classification, Demographic Characteristics, and Background
Five hundred and seventy-one patients were included in the study. Of these, 317 (56%) were from the centers in the state of Pernambuco, 139 (24%) from Rio Grande do Norte, and 115 (20%) from Paraíba. Of the total cohort, 355 (62%) had a diagnosis of UC, and 216 (38%) had a diagnosis of CD. Most of the patients were women (355, 62%), corresponding to 64.5% and 58.3% of UC and CD cases, respectively (p=0.165). The ages ranged from 15 to 100 years. The mean age ± standard deviation in the CD group was 39.7 years ± 14.9 years and the median age was 38.5 years; in the UC group, the mean age was 45.8 years ± 16.5 years and the median age was 47 years (p<0.0001).
Regarding race, most of the patients were non-white, representing 63.9% of the cases. Mixed-race patients accounted for 47.6% of the cases, followed by white patients (36.1%), black patients (15.6%), and Indian patients (0.7%). In the CD group, there was a higher frequency of white patients (43.1%) than in the UC group (31.8%) (p=0.008).
In the evaluation of family income, and based on the monthly minimum wage of $205 in the region, most patients (53.9%) earned between 1 and 2 salaries, followed by patients who earned between 2 and 4 salaries (19.3%), those living on less than 1 salary (9.8%), those with income between 4 and 6 salaries (3.7%), and those with more than 6 salaries (2.1%). Those who declined to report their earnings accounted for 11.2% of the total.
For education, most patients had up to 12 years of schooling (80.2%), with 43.8% of these having completed high school (12 years). The demographic characteristics are detailed in Table 1.
 |
Table 1 Demographic Profile of Patients with Inflammatory Bowel Disease at Reference Centers in Three States of Northeast Brazil |
Regarding the place of residence, 199 (34.8%) patients lived in the capitals of the respective states. This proportion did not differ significantly among the three states or between the diseases.
A history of smoking was reported by 20.4% of the CD patients and 34.9% of the UC patients (p=0.0003). However, at the end of the study, only 4.2% and 4.5% of the CD and UC cases, respectively, still smoked.
A total of 12% of the patients reported a family history of IBD (CD and UC).
Past appendectomy was reported by 14 (6.5%) and 4 (1.1%) patients with CD and UC, respectively (p=0.001).
Temporal Analysis
When evaluating the number of diagnosed cases of IBD over time, we observed a progressive increase in newly diagnosed cases in every 5-year period over the past four decades. The greatest increases were recorded in the 2011–2015 period, representing 25% of the total, and the 2016–2020 period when 34% of the cases were diagnosed (Figure 1). It was observed that over time, inflammatory bowel disease presented an average growth rate of 0.81 per year with statistical significance (p < 0.0001) (Figure 2).
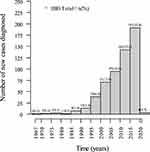 |
Figure 1 Number of newly diagnosed cases every five years in patients with inflammatory bowel disease at reference centers in three states of Northeast Brazil. Abbreviation: IBD, inflammatory bowel disease. |
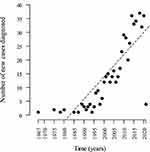 |
Figure 2 Trend and frequency of newly diagnosed cases observed that over time in patients with inflammatory bowel disease at reference centers in three states of Northeast Brazil. It was observed that over time, inflammatory bowel disease presented an average growth rate of 0.81 per year with statistical significance (p < 0.0001). |
Clinical Profile
The time between the onset of symptoms and diagnosis was 27.4 ± 45.7 years (mean ± standard deviation), with a median of 12 months, for CD; and 19.4 ± 38.7 years and 8 months, respectively, for UC (p=0.012). Most patients with CD were diagnosed between the ages of 17 and 40, corresponding to 60.2% of this group, followed by the over-40 age group, accounting for 23.15%. Among UC patients, the percentages for the same age groups were 52.7% and 37.2%, respectively (p=0.0008).
In CD, ileocolonic involvement (L3) was the predominant manifestation, occurring in 82 (38%) patients, followed by colonic disease (L2), diagnosed in 65 (30.1%) patients, and ileal disease (L1), detected in 57 (26.4%) patients. Twelve cases (5.6%) had upper gastrointestinal (L4) tract involvement, with 10 cases (4.7%) having simultaneous lower gastrointestinal tract involvement and two cases occurring only L4 (0.9%). Regarding the behavior of the disease, 85 (39.4%) cases were classified as B1 (inflammatory), 89 (41.2%) displayed a stenosing pattern (B2), and 42 (19.4%) exhibited penetrating (B3) behavior. Perineal disease (p) was present in 15 (17.6%), 19 (21.3%), and 19 (45.2%) patients, with patterns of inflammatory (B1), penetrating (B3), and stenosing disease (B2), respectively (Table 2).
 |
Table 2 Montreal Classification of Patients with Inflammatory Bowel Disease at Reference Centers in Three States of Northeast Brazil |
In UC, 101 (28.4%) of the cases had an E1 pattern (proctitis), 116 (32.7%) had an E2 pattern (left-sided colitis), and 138 (38.9%) had an E3 pattern (extensive colitis). At the time of recruitment, 182 (51.3%) diseases were classified as S0 (no symptoms, disease in clinical remission), 78 (21,9%) as S1 (mild symptoms), 71 (20%) as S2 (moderate), and 24 (6.8%) as S3 (severe) (Table 2).
Extraintestinal Manifestations
Of the extraintestinal manifestations, articular manifestations were the most frequent. Arthralgia was reported by 241 (42.2%) patients and was more frequent among patients with UC (44.2%) than in those with CD (38.9%). Meanwhile, arthritis was described in 107 (18.7%) patients, and was diagnosed more frequently in UC patients (20.8%) than in CD patients (15.3%); however, the differences were not significant.
Nine patients (1.6%) reported a diagnosis of spondylitis and four (0.7%) had a diagnosis of sacroiliitis, but the differences between the groups were not significant. When asked about their history of lower back pain, lumbar morning stiffness, and night awakening due to lower back pain, the frequency of positive responses was 31%, 11.9%, and 11.4%, respectively. These responses were reported by respectively 26.4%, 9.26%, and 11.1% of CD patients and 34.4%, 13.8%, and 11.8% of patients with UC. The presence of at least two of these complaints was reported in 97 (16.9%) patients, specifically, in 13.9% and 18.9% of the patients with CD and UC, respectively (p<0.1547).
Of the cutaneous manifestations, pyoderma gangrenosum was present in 12 (3.4%) cases of UC and 6 (2.8%) of CD, while erythema nodosum occurred in 5 (1.4%) and 4 (1.9%) of the cases of UC and CD, respectively; however, the difference between the groups was not significant.
Uveitis was diagnosed in 15 (2.6%) patients, and another 15 (2.6%) were diagnosed with episcleritis, with no significant differences between the UC and CD groups.
Primary sclerosing cholangitis was diagnosed in 26 patients (4.6%), 26 (7.3%) in the UC group and none in the CD group (p=0.0037).
Deep venous thrombosis was reported in 5 (1.4) patients with UC and 1 (0.5%) with CD. Table 3 summarizes the extraintestinal manifestations of this cohort.
 |
Table 3 Extraintestinal Manifestations in Patients with Inflammatory Bowel Disease at Reference Centers in Three States of Northeast Brazil |
Pharmacological and Surgical Treatment
In the evaluation of therapy by drug class, 157 (72.7%) patients with CD were using biological therapy; in 83 (52.9%) of these cases, biologicals were administered in combination with an immunosuppressant, either azathioprine or methotrexate. Immunosuppressants without biological therapy were used by 42 (19.4%) patients, 7 (16.7%) of whom also used aminosalicylates (Table 4).
 |
Table 4 Therapy Used in Patients with Inflammatory Bowel Disease at Reference Centers in Three States of Northeast Brazil |
In the UC group, aminosalicylates were the most prescribed drugs (used by 192 [54.1%] patients), followed by biologicals, taken by 93 (26.2%) patients, 33 (35.5%) of whom were also taking immunosuppressants at the time of enrolment. Immunosuppressants without biological agents were used by 54 (15.2%) patients, 40 (74.1%) of whom used them in combination with aminosalicylates (Table 4).
Resection surgery was performed in 57 (26.4%) of the CD patients and 16 (4.5%) of the UC patients (p<0.0001). Fistulectomy and/or seton placement was performed in 59 (27.3%) patients with CD (Table 4).
Discussion
In this study encompassing three states in Northeast Brazil, we observed a progressive increase in the diagnosis of IBD cases in specialized units within every 5-year period over the last five decades, with 58.6% of cases being diagnosed in the last 10 years. Other studies have also reported a progressive increase in the prevalence of IBD in Brazil.10,12,16,17 In this study encompassing three states in Northeast Brazil, we observed a progressive increase in the diagnosis of IBD cases in specialized units within every 5-year period over the last four decades, with 58.6% of cases being diagnosed in the last 10 years. Temporal analysis based on the diagnosis of new cases in healthcare units has been used as an indirect assessment of case increases in a region.18–21 In Brazil, this progressive increase in newly diagnosed cases over the years has been reported in studies from the southeast and northeast regions, but IBD-related data for the Northeast region are limited.18,21,22 A trend of increasing newly diagnosed cases over the years in outpatient units has been reported in other Latin American and Asian countries, historically considered to have low prevalence of IBD.19,20 Despite the methodology not allowing for population extrapolations, this increase has been described using other methodologies employed in Brazil, reinforcing that the rise in newly diagnosed cases in healthcare units may reflect the increase in population cases.12 Based on a public database of medication dispensing data for patients with IBD, Quaresma et al reported that the IBD prevalence increased from 30.0 cases per 100,000 inhabitants in 2012 to 100.1 cases per 100,000 inhabitants in 2020 in Brazil, considering aggregated data from the entire country.12 The rise in IBD prevalence may be associated with the progressive increase in industrialization/urbanization, which leads to improved hygienic conditions and lifestyle changes and, consequently, greater exposure to risk factors for IBD, such as changes in diets, increased smoking, and less physical activity.13,23–25 Official data show that there has been a progressive increase in urbanization in the Northeast region of Brazil.26 Greater access to the health care system and diagnostic methods may have also contributed to this increase in IBD cases.
No correlation was found between ethnicity and IBD in this study. Most of our cohort were of mixed race, with white patients accounting for 36% of the total, similar to the racial distribution of the population of the region. The prevalence of IBD is higher among Caucasians than among other ethnic groups; however, an increase in cases of the disease has also been reported in countries with different ethnicities.10,11 Similar results were found in two other states of Northeastern Brazil, Piauí and Bahia, where mixed-race individuals accounted for 68% and 56% of the population, respectively.22,27
Studies have reported differences in the gender distribution of IBD, with equal frequencies in European and North American countries, and a higher frequency in men in the Asia–Pacific region.5–7,28 In the present study, females predominated, accounting for 58.3% and 64.5% of CD and UC cases, respectively. These data are similar to those described in Latin American countries and some Brazilian states.17,18,29–32
In the present study, the median time between the onset of symptoms and diagnosis was 12 months for CD and 8 months for UC (p=0.042). In developed countries, the median time to diagnosis is less than 12 months.33–35 In a multicentre study in Italy, the median time to diagnosis was reported to be 7.1 months for CD and 2 months for UC.33 In a cohort study conducted by Burisch et al involving 31 centers in Eastern and Western European countries, the median time was less than 5 months for CD and 3 months for UC.34 In Brazil, few studies have evaluated these intervals; however, the reported values have differed between publications. A study conducted by Silva et al in Northeast Brazil, reported a median time to diagnosis of 20.4 months for UC, while another study in São Paulo in Southeast Brazil, a region with indicators of a developed country, the median was 20 months for CD and 11 for UC.22,29 The longer time to diagnosis for CD and UC in less developed regions may be related to a greater precariousness of the health care system available to the population, with fewer reference services, less access to diagnostic tests, and fewer qualified professionals relative to developed regions. However, when comparing our results with those of a study undertaken in 2012 involving one of the centers participating in this study, we observe a reduction in the median time to diagnosis over time (48 vs 12 months for CD and 12 vs 8 months for UC).31 We attribute this reduction to progressive regional economic and social development, the creation of new services specializing in inflammatory diseases, and greater access and adherence of professionals to information and investigative protocols over recent years, especially in the last two decades, the period in which the greatest number of IBDs was diagnosed in our cohort.
Smoking habits at the time of inclusion in the study were similar between the CD and UC groups, with approximately 4% of the patients being active smokers. When evaluating past smoking habits, the frequency of smoking was higher in the UC group (40%) than in the CD group (20%), similar to that reported in other Latin American countries. Smoking has been associated with a higher risk for CD, whereas smoking cessation has been linked to an increase in the incidence and severity of UC, an effect that may persist for decades.5,36,37 In the present study, despite there being a greater percentage of smoking cessation among UC patients than among CD patients, we did not assess whether smoking cessation preceded the onset of the disease. A study by da Silva et al in a different state in the northeast of the country reported frequencies of active smoking and past smoking habits of 3% and 34.5%, respectively, similar to those found in the present study.22
A family history of IBD was present in approximately 12% of the patients with UC and CD, with a prevalence equal to that reported in a meta-analysis of 71 studies from Asia, Europe, and the USA.38 In two studies in Northeast Brazil, the frequency of family history of IBD was 7.9% and 11.5%, respectively, the latter being similar to that reported here.22,31
Most patients with IBD were diagnosed with the disease between the ages of 17 and 40, corresponding to an economically active stage of life. Accordingly, IBD diagnosis may be associated with socioeconomic impacts, such as compromised professional and educational activities and interpersonal relationships. These findings are similar to those reported in European countries and the USA, as well as in other regions of Brazil.18,19,31 However, different patterns have been described in Asia. For example, in a meta-analysis of a Chinese population, Li et al found that 64% of UC cases were diagnosed in patients over the age of 40.39 In a review of epidemiological studies in the Asia–Pacific region, Ng et al reported a disease pattern for CD comprising two age-at-diagnosis peaks, the first between 20 and 24 years and the second between 40 and 44 years.28
Regarding disease behavior, we found a predominance of ileocolonic involvement (L3) in patients with CD (38% of the cases), with stenosing (B2) and/or penetrating (B3) behaviour present in 131 (60.6%) of these cases. These disease patterns are frequently associated with complications. In a study involving countries in the Asia–Pacific region, Ng et al also reported a predominance of ileocolonic disease (L3); however, most of the patients displayed a B1 inflammatory pattern.28 In contrast, in another study conducted by Jiang et al in Wuhan, China, 70% of patients presented with stenosing or penetrating disease.20 In Europe, non-penetrating and non-stenosing (B1) behaviors are the most frequently described. Burisch et al reported that more than 60% of patients had pattern B1.34 Our findings also differ from those of Parente et al in Northeast Brazil, where the inflammatory pattern (B1) predominated (69% of the cohort), and colonic (L1) involvement accounted for 36% of the cases.31 Our results are similar to those described in São Paulo, Southeast Brazil, where most cases involved complex disease and only 30% exhibited a B1 pattern.21
In UC, extensive colitis (E3) predominated in our study and was present in 38.9% of the cases. This was in contrast to that reported in Asia and Europe, where a predominance of left-sided disease (E3) is described.19,20,28,34 Data from studies in Northeast Brazil also differ from those of the present study. In Piauí, Parente et al reported that 61% of cases had an E2 pattern (left-sided colitis), while in Bahia, Silva et al also found that E2 disease (left-sided colitis) was the most frequent pattern, but with a frequency of less than 40%. In Sergipe, meanwhile, Delmondes et al reported a predominance of the E1 (proctitis) pattern (71% of cases).22,29,31 Our results are also inconsistent with those of other regions of Brazil. In a study by Martins et al in Minas Gerais, Southeast Brazil, the authors reported a predominance of the E1 pattern, accounting for 40% of the sample.18 However, the results of a study by Gomes et al in São Paulo were similar to those in our study, where a predominance of extensive colitis (E3) was found and was present in 50% of the sample.21
In the present study, joint involvement was the most frequent extraintestinal manifestation, with arthralgia and arthritis found to be present in 42% and 19% of the patients, respectively. Based on the literature, arthritis affects between 10% and 15% of IBD patients; however, as many as 50% of patients suffer from noninflammatory joint pain.40–42 Relatively few studies have evaluated these manifestations in studies conducted in Northeast Brazil. Demondes reported a frequency of 35% and 30% for arthralgia and arthritis in CD, respectively, while in a study in Bahia, Silva et al reported a frequency of 34% for joint involvement among UC cases but did not specify the type of manifestation.22,29
Based on the standardized questionnaire of Queiro et al,15 23% of our cohort had symptoms compatible with peripheral spondyloarthritis, and 17% had symptoms compatible with axial spondyloarthritis. The treatment of these joint manifestations requires a multidisciplinary approach, as well as early identification and therapy. The inclusion of standardized and systematic questions may help gastroenterologists detect cases of spondyloarthritis. Congliaro et al showed that there was a delay in the diagnosis of these diseases by gastroenterologists.43 Their study, involving 269 patients with joint pain, found that, as evaluated by a rheumatologist, 50% of the cases met the criteria for the diagnosis of peripheral spondyloarthritis, while 20% met the criteria for the diagnosis of axial spondyloarthritis, with a mean delay of 5 years in the diagnosis of axial disease.43
Regarding therapy, there was a high frequency of the use of biologicals among our cohort, with 73% of the patients using them; however, this figure dropped to 26% among patients with UC. Relatively few studies have been published in recent years detailing the therapeutic approach for the treatment of patients with IBD in Brazil.18,21 In Northeast Brazil, one study reported a 1.5% rate of use of biologics among 265 patients with UC; however, this study analyzed patients only until 2012, when access to biologics in the region was limited.22 In the state of São Paulo, a study involving 303 patients with CD and 355 with UC reported the use of anti-TNF agents in 56% and 18.6% of the patients, respectively.21 In a study conducted in Central-West Brazil in a private health institution involving 329 patients with IBD, anti-TNF therapy was prescribed in 62.7% and 44.4% of patients with CD and UC, respectively.44 However, in the same region, another study involving 423 patients from a public health service reported a lower frequency of biological therapy prescription, with values of 53.8% and 10.8% for CD and UC, respectively.45 The high frequency of the use of biologics in our service may be related to a large number of patients with severe disease being referred to the centers participating in the study, which are reference centers for IBD in the Northeast of Brazil, hat use recommendations from international guidelines for the use of biological therapy.2–4,18,21
In our study, anti-TNF drugs accounted for 87% of the biologics prescribed, especially infliximab, which was prescribed in 59% of these cases. These drugs were the only biologics available in the public health system of Brazil for many years. Other biological drugs, such as ustekinumab and vedolizumab, were only made available from 2019, and then only for patients with UC. Few patients were prescribed these drugs for CD, and only following legal action.
Aminosalicylates were the drugs most prescribed to patients with UC, corresponding to 54% of the sample, followed by azathioprine, used by 24% of UC patients. However, aminosalicylate prescription corresponded to only 15% of the sample when excluding their use in combination with biologics, with a prescriptive pattern similar to that reported in other studies and following international guidelines.1–4,20,21,46 In the present study, 13.5% of the patients with UC who were using immunosuppressants also used aminosalicylates. Although this combination does not form part of the guidelines, it is still prescribed in clinical practice. However, in a literature review published in 2020, the American Gastroenterological Association stressed the lack of scientific evidence justifying the use of this combination therapy.47 The American Gastroenterological Association guidelines state that evidence for a potential benefit of the long-term use of 5-ASA for chemoprevention against colorectal cancer is limited, and does not justify the association, suggesting evidence that the risk of neoplasia is related to active disease, with sustained remission being the most important protective factor against colorectal cancer, regardless of the therapy that achieves this result.47 These results found in our study allowed reflection on adjustments in the routines of our services based on the review of the best evidence.
In the present study, approximately 20% of the CD patients and 5% of the UC patients underwent surgical resection, similar to the rates reported in the literature. Surgery has become rarer in recent decades with the advent of novel biological therapies.6,7,48 Vegh et al reported that the cumulative probability of intestinal surgery for CD decreased from 59% in patients diagnosed between 1986 and 1991 to 25% in patients diagnosed between 1998 and 2003.6 In São Paulo, Gomes et al reported a higher frequency of surgery among patients with CD than that observed in our study (52.8% vs 20%); however, the frequency of surgery for UC (5%) was the same in both studies.21
Conclusion
This is the first multicentre prospective study conducted in states in Northeast Brazil, a region with reduced socio-economic development compared with other regions of the country. Our study allowed for the regular follow-up of patients via the application of a standardized questionnaire, which made it possible to describe the different variables with greater accuracy than that possible with retrospective studies or studies employing secondary databases, which are less complete and subject to differences in data gathering. We observed a progressive increase in the number of newly diagnosed cases of IBD in the last 10 years, which may be related to the progressive economic and social development of the region. Some results, such as the predominance of UC and women among patients with IBD, differ from those reported in developed countries in Europe and North America. The predominant age group at diagnosis (17–40 years) comprises an economically active population, which increases the subsistence challenges in the region for the families of these patients. Disease behavior was mostly characterized as extensive, difficult to control, and frequently associated with complications, which may be partly due to the prolonged time between the onset of symptoms and diagnosis of the disease. The high frequency of biological therapy, especially among CD patients, reinforces the pattern found. Joint involvement was more frequent than described in other countries and in other regions of Brazil, which may be explained, at least in part, by the systematic manner in which these manifestations were investigated in our study. This may suggest the need to use standardized questionnaires to identify these extra-intestinal manifestations, avoiding delays in diagnosis, and use of specific therapies. We believe that the publication of regional data will help to raise the awareness of health professionals regarding the relevance of the disease. Disseminating information relating to the increase in cases of IBD in the region and its clinical–epidemiological patterns, complications, available therapies, and the need for early diagnosis may contribute to better management of cases in the health service.
Data Sharing Statement
All data generated or analyzed during this study are included in the published article.
Ethics Approval and Consent to Participate
Written informed consent for publication was obtained from the patients before information was collected. The patients also provided written consent for the inclusion of personal and clinical details in this study. The study was approved by the Research Ethics Committee of the Centre of Medical Sciences, Federal University of Pernambuco (CAAE [certificate of presentation of ethical appreciation]: 31047320.7.0000.5208).
Consent for Publication
The patients provided written, informed consent for the inclusion of personal and clinical details in this study.
Acknowledgments
We thank the Autoimmune Institute for Research and Continuing Education for its support in the development of this project.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Takeda Pharmaceuticals, Brazil, under a specific grant (IISR-2020-102789). The sponsors did not participate in the design of the study, the analysis of the results, or the content of the publication.
Disclosure
Dr Lívia Medeiros Soares Celani reports grants from Takeda Pharmaceutical Company, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Ko CW, Singh S, Feuerstein JD, et al. AGA clinical practice guidelines on the management of mild-to-moderate ulcerative colitis. Gastroenterology. 2019;156(3):748–764. doi:10.1053/j.gastro.2018.12.009
2. Feuerstein JD, Isaacs KL, Schneider Y, et al. AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020;158(5):1450–1461. doi:10.1053/j.gastro.2020.01.006
3. Raine T, Bonovas S, Burisch J, et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis. 2022;16(1):2–17. doi:10.1093/ecco-jcc/jjab178
4. Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2020;14(1):4–22. doi:10.1093/ecco-jcc/jjz180
5. Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1785–1794. doi:10.1053/j.gastro.2011.01.055
6. Vegh Z, Kurti Z, Lakatos PL. Epidemiology of inflammatory bowel diseases from west to east. J Dig Dis. 2017;18(2):92–98. doi:10.1111/1751-2980.12449
7. Mak WY, Zhao M, Ng SC, Burisch J. The epidemiology of inflammatory bowel disease: east meets west. J Gastroenterol Hepatol. 2020;35(3):380–389. doi:10.1111/jgh.14872
8. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies [published correction appears in Lancet. 2020 Oct 3;396(10256):e56]. Lancet. 2017;390(10114):2769–2778. doi:10.1016/S0140-6736(17)32448-0
9. Ng SC, Kaplan GG, Tang W, et al. Population density and risk of inflammatory bowel disease: a prospective population-based study in 13 countries or regions in Asia-Pacific. Am J Gastroenterol. 2019;114(1):107–115. doi:10.1038/s41395-018-0233-2
10. Selvaratnam S, Gullino S, Shim L, et al. Epidemiology of inflammatory bowel disease in South America: a systematic review. World J Gastroenterol. 2019;25(47):6866–6875. doi:10.3748/wjg.v25.i47.6866
11. Park J, Cheon JH. Incidence and prevalence of inflammatory bowel disease across Asia. Yonsei Med J. 2021;62(2):99–108. doi:10.3349/ymj.2021.62.2.99
12. Quaresma AB, Damiao AOMC, Coy CSR, et al. Temporal trends in the epidemiology of inflammatory bowel diseases in the public healthcare system in Brazil: a large population-based study. Lancet Reg Health Am. 2022;13:100298. doi:10.1016/j.lana.2022.100298
13. Du L, Ha C. Epidemiology and pathogenesis of ulcerative colitis. Gastroenterol Clin North Am. 2020;49(4):643–654. doi:10.1016/j.gtc.2020.07.005
14. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a working party of the 2005 montreal world congress of gastroenterology. Can J Gastroenterol. 2005;19(Suppl A):5A–36A. doi:10.1155/2005/269076
15. Queiro R, Rodríguez-Caminero S, Riestra S, de Francisco R, Pérez-Martínez I, Ballina J. Performance of two screening questionnaires for inflammatory arthritis in patients with inflammatory bowel disease. Biomed Res Int. 2018;2018:8618703. doi:10.1155/2018/8618703
16. Gasparini RG, Sassaki LY, Saad-Hossne R. Inflammatory bowel disease epidemiology in São Paulo State, Brazil [published correction appears in Clin Exp Gastroenterol. 2020 Jun 16;13:221]. Clin Exp Gastroenterol. 2018;11(11):423–429. doi:10.2147/CEG.S176583
17. Kotze PG, Steinwurz F, Francisconi C, et al. Review of the epidemiology and burden of ulcerative colitis in Latin America. Therap Adv Gastroenterol. 2020;13:1756284820931739. doi:10.1177/1756284820931739
18. Martins KR, Araújo JM, Ác C, Luiz-Ferreira A. Epidemiologic aspects of inflammatory bowel disease in the western region of Minas Gerais state. Arq Gastroenterol. 2021;58(3):377–383. doi:10.1590/S0004-2803.202100000-63
19. Lucendo AJ, Hervías D, Roncero Ó, et al. Epidemiology and temporal trends (2000–2012) of inflammatory bowel disease in adult patients in a central region of Spain. Eur J Gastroenterol Hepatol. 2014;26(12):1399–1407. doi:10.1097/MEG.0000000000000226
20. Jiang L, Xia B, Li J, et al. Retrospective survey of 452 patients with inflammatory bowel disease in Wuhan city, central China. Inflamm Bowel Dis. 2006;12(3):212–217. doi:10.1097/01.MIB.0000201098.26450.ae
21. Gomes TNF, de Azevedo FS, Argollo M, Miszputen SJ, Ambrogini O
22. da Silva BC, Lyra AC, Mendes CMC, et al. The demographic and clinical characteristics of ulcerative colitis in a Northeast Brazilian population. Biomed Res Int. 2015;2015:359130. doi:10.1155/2015/359130
23. Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18(1):56–66. doi:10.1038/s41575-020-00360-x
24. Soon IS, Molodecky NA, Rabi DM, Ghali WA, Barkema HW, Kaplan GG. The relationship between urban environment and the inflammatory bowel diseases: a systematic review and meta-analysis. BMC Gastroenterol. 2012;12(1):51. doi:10.1186/1471-230X-12-51
25. Li T, Qiu Y, Yang HS, et al. Systematic review and meta-analysis: association of a pre-illness Western dietary pattern with the risk of developing inflammatory bowel disease. J Dig Dis. 2020;21(7):362–371. doi:10.1111/1751-2980.12910
26. Departamento de informática do Sistema Único de Saúde do Brasil. Brasil: Ministério da Saúde. Available from:
27. Oliveira TC, Lima MM, Coelho CD, et al. Perfil clínico-epidemiológico de pacientes com Doença Inflamatória Intestinal internados no Hospital Universitário da Universidade Federal do Piauí [CLINICAL-EPIDEMIOLOGICAL PROFILE OF PATIENTS WITH INFLAMMATORY BOWEL DISEASE HOSPITALIZED AT THE UNIVERSITY HOSPITAL OF THE FEDERAL UNIVERSITY OF PIAUÍ]. Jornal de Ciências da Saúde do Hospital Universitário da Universidade Federal do Piauí. 2018;1(1):34. doi:10.26694/2595-0290.1134-40
28. Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific crohn’s and colitis epidemiology study. Gastroenterology. 2013;145(1):158–165.e2. doi:10.1053/j.gastro.2013.04.007
29. Delmondes LM, Nunes MO, Azevedo AR, Oliveira MM, Coelho LE, Torres-Neto JD. Clinical and sociodemographic aspects of inflammatory bowel disease patients. Gastroenterol Res. 2015;8(3–4):207–215. doi:10.14740/gr649w
30. Dall’Oglio VM, Balbinot RS, Muscope AL, et al. Epidemiological profile of inflammatory bowel disease in Caxias do Sul, Brazil: a cross-sectional study. Sao Paulo Med J. 2020;138(6):530–536. doi:10.1590/1516-3180.2020.0179.r2.10092020
31. Parente JM, Coy CS, Campelo V, et al. Inflammatory bowel disease in an underdeveloped region of Northeastern Brazil. World J Gastroenterol. 2015;21(4):1197–1206. doi:10.3748/wjg.v21.i4.1197
32. Santos RM, Carvalho AT, Silva KD, et al. Inflammatory bowel disease: outpatient treatment profile. Arq Gastroenterol. 2017;54(2):96–100. doi:10.1590/s0004-2803.201700000-01
33. Cantoro L, Di Sabatino A, Papi C, et al. The time course of diagnostic delay in inflammatory bowel disease over the last sixty years: an Italian multicentre study. J Crohns Colitis. 2017;11(8):975–980. doi:10.1093/ecco-jcc/jjx041
34. Burisch J, Pedersen N, Čuković-čavka S, et al. East-west gradient in the incidence of inflammatory bowel disease in Europe: the ECCO-EpiCom inception cohort. Gut. 2014;63(4):588–597. doi:10.1136/gutjnl-2013-304636
35. Vavricka SR, Spigaglia SM, Rogler G, et al. Systematic evaluation of risk factors for diagnostic delay in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18(3):496–505. doi:10.1002/ibd.21719
36. Beaugerie L, Massot N, Carbonnel F, Cattan S, Gendre JP, Cosnes J. Impact of cessation of smoking on the course of ulcerative colitis. Am J Gastroenterol. 2001;96(7):2113–2116. doi:10.1111/j.1572-0241.2001.03944.x
37. Higuchi LM, Khalili H, Chan AT, Richter JM, Bousvaros A, Fuchs CS. A prospective study of cigarette smoking and the risk of inflammatory bowel disease in women. Am J Gastroenterol. 2012;107(9):1399–1406. doi:10.1038/ajg.2012.196
38. Childers RE, Eluri S, Vazquez C, Weise RM, Bayless TM, Hutfless S. Family history of inflammatory bowel disease among patients with ulcerative colitis: a systematic review and meta-analysis. J Crohns Colitis. 2014;8(11):1480–1497. doi:10.1016/j.crohns.2014.05.008
39. Li X, Song P, Li J, et al. The disease burden and clinical characteristics of inflammatory bowel disease in the Chinese population: a systematic review and meta-analysis. Int J Environ Res Public Health. 2017;14(3):238. doi:10.3390/ijerph14030238
40. De Brito CA. The most relevant extraintestinal manifestations of inflammatory bowel disease: a multidisciplinary approach. Gastroenterol Hepatol Res. 2021;6(1). doi:10.24966/ghr-2566/100036
41. Park SK, Wong Z, Park SH, et al. Extraintestinal manifestation of inflammatory bowel disease in Asian patients: a multinational study. Dig Liver Dis. 2021;53(2):196–201. doi:10.1016/j.dld.2020.06.046
42. Algaba A, Guerra I, Ricart E, et al. Extraintestinal manifestations in patients with inflammatory bowel disease: study based on the ENEIDA registry. Dig Dis Sci. 2021;66(6):2014–2023. doi:10.1007/s10620-020-06424-x
43. Conigliaro P, Chimenti MS, Ascolani M, et al. Impact of a multidisciplinary approach in enteropathic spondyloarthritis patients. Autoimmun Rev. 2016;15(2):184–190. doi:10.1016/j.autrev.2015.11.002
44. Cury DB, Oliveira R, Cury MS. Inflammatory bowel diseases: time of diagnosis, environmental factors, clinical course, and management – a follow-up study in a private inflammatory bowel disease center (2003–2017). J Inflamm Res. 2019;12:127–135. doi:10.2147/JIR.S190929
45. Arantes JA, Santos CH, Delfino BM, et al. Epidemiological profile and clinical characteristics of patients with intestinal inflammatory disease. J Coloproctology. 2017;37(4):273–278. doi:10.1016/j.jcol.2017.06.004
46. Lamb CA, Kennedy NA, Raine T, et al. British society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults [published correction appears in Gut. 2021 Apr;70(4):1]. Gut. 2019;68(Suppl 3):s1–s106. doi:10.1136/gutjnl-2019-318484
47. Singh S, Allegretti JR, Siddique SM, Terdiman JP. AGA technical review on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020;158(5):1465–1496.e17. doi:10.1053/j.gastro.2020.01.007
48. Park SJ, Kim WH, Cheon JH. Clinical characteristics and treatment of inflammatory bowel disease: a comparison of eastern and western perspectives. World J Gastroenterol. 2014;20(33):11525–11537. doi:10.3748/wjg.v20.i33.11525
